Pharma LBL Design


Gemcitabine Hydrochloride Injection LBL
LBL full form in pharma
In pharma marketing terms, LBL stands for Leave-Behind Literature. The medical representative leaves the LBL at the doctor's table. So it's called leave-behind literature.
LBL (leave-behind literature) is a key tool in pharma marketing. While reps take back visual aid after presentations, LBLs stay with doctors for future reference. Since doctors see many brands daily, a well-designed, informative LBL helps them remember yours—but only if it’s engaging and up-to-date.
Why Our LBLs Work:
✅Latest Research—Fresh clinical data, studies, and references
✅ Easy to Understand—Clear graphs, infographics, and key takeaways
✅ Visually Engaging—Professional design that holds attention
✅ Action-Oriented—Helps doctors recall your brand when prescribing
We specialize in creating high-impact LBLs that doctors keep and use. Let’s make sure your message sticks—without ending up in the trash.
Trust us to deliver literature that leaves a lasting impression and supports your messaging goals.
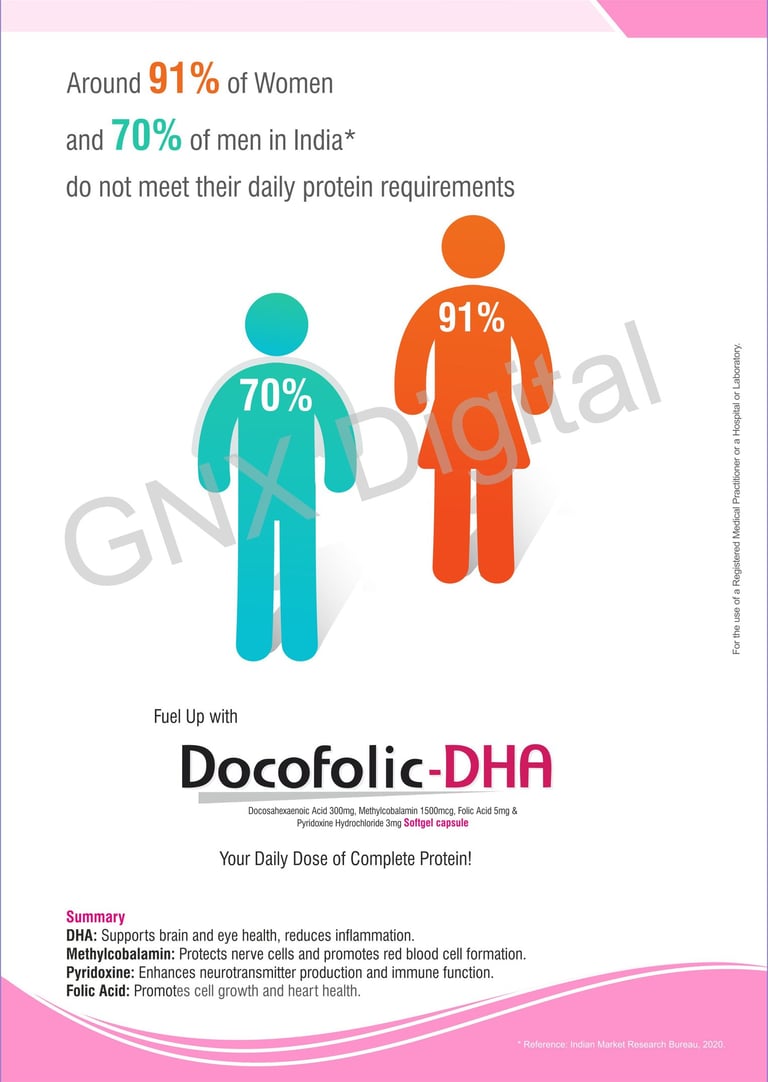
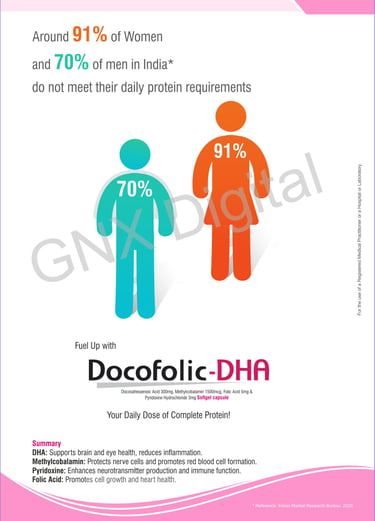
Creative Pharma LBL design




LBL Design Sample


Frequently asked questions
Q1: What is the full form of LBL?
A: LBL full form in pharma is leave-behind literature. It is a marketing and communication tool used by pharmaceutical companies and representatives to provide detailed information about their products, services, or medical concepts to healthcare professionals (HCPs).
Q2: What is the purpose of LBLs?
A: Pharma LBLs are designed to educate and inform HCPs about a pharmaceutical product's key features, benefits, clinical evidence, and usage guidelines. They serve as a quick reference and reinforcement of a product's value after a sales visit or meeting.
Q3: What kind of information is typically included in Pharma LBLs?
A: Pharma LBLs generally include:
Product overview (indications, dosage, administration).
Clinical trial results and scientific data.
Mechanism of action (MoA) visuals.
Safety and efficacy information.
Patient benefits and case studies.
Key references and regulatory approvals.
Q4: Who are the primary recipients of Pharma LBLs?
A: Pharma LBLs are primarily intended for healthcare professionals such as doctors, pharmacists, nurses, and specialists who prescribe or recommend pharmaceutical products.
Q5: How are Pharma LBLs designed?
A: Pharma LBLs are crafted to be concise, visually appealing, and informative. They often feature charts, graphs, and infographics to present scientific data. The design focuses on delivering key messages effectively and ensuring compliance with medical and regulatory standards.
Q6: What makes an effective Pharma LBL?
A: An effective Pharma LBL should:
Be clear, concise, and easy to understand.
Include accurate and up-to-date scientific data.
Comply with regulatory guidelines.
Have a professional and engaging design.
Address the specific needs and concerns of the target audience.
Q7: Are Pharma LBLs used globally?
A: Yes, Pharma LBLs are widely used worldwide. However, the content and design may vary to meet regional regulatory requirements and cultural preferences.
Q8: How does Pharma LBL differ from other promotional materials?
A: Unlike general advertising or promotional brochures, Pharma LBLs focus on providing detailed, evidence-based information tailored for healthcare professionals. They are less about broad promotion and more about fostering informed decision-making.
Q9: Can Pharma LBLs be digital?
A: Yes, in addition to traditional printed formats, Pharma LBLs can be shared digitally through PDFs, emails, or interactive platforms, offering a more flexible and eco-friendly option.
Q10: Why are Pharma LBLs important?
A: Pharma LBLs are crucial in bridging the gap between pharmaceutical companies and healthcare providers. They enhance understanding, support informed prescribing decisions, and ultimately contribute to improved patient care.
Socials
Subscribe to our newsletter
Copyright © 2026. All right reserved—gnxdigital.com
